Lithium-ion batteries are closely related to our daily life, however, the traditional lithium batteries contain a large amount of organic liquid electrolyte, which has the safety hazard of fire and even explosion, and the low energy density makes it difficult to meet the requirements of emerging strategic industries such as aerospace and new energy vehicles. The solid-state battery developed by using solid electrolyte instead of organic electrolyte is a new type of energy storage technology, which is expected to solve the safety problems of traditional lithium batteries and improve the energy density of batteries, and is expected to be applied in the field of new energy vehicles, and has received wide attention. As the core material of solid-state batteries, it is very important and challenging to develop solid-state electrolyte materials with high ionic conductivity at room temperature, high ion mobility, and good compatibility with metal anode and high-voltage cathode.
Recently, Professor Ning Hu, collaborated with Associate Professor Shufeng Song from Chongqing University and Professor Yong Yang from Xiamen University, has designed a "Superconcentrated Ionogel-in-Ceramic" (SIC) solid-state electrolyte by an-in situ thermally initiated radical polymerization reaction. The electrolyte not only exhibits an ultra-high room temperature ion conductivity (1.33 × 10-3 S cm-1), but also has an ultra-high lithium ion transference number of 0.89 and an electrochemical window of 5.5 V. The solid-state battery matched with lithium metal cathode and NCM523 and LiFePO4 exhibits excellent cycling stability. The related work was published as "Enabling High-Voltage "Superconcentrated Ionogel-in-Ceramic " Hybrid Electrolyte with Ultrahigh Ionic Conductivity and Single Li+-ion Transference Number" in the internationally top journal Advanced Materials (DOI: 10.1002/adma.202205560). The first author is Yanfang Zhai, a PhD student from Chongqing University, and the corresponding authors are Professor Ning Hu, Shufeng Song and Yong Yang.
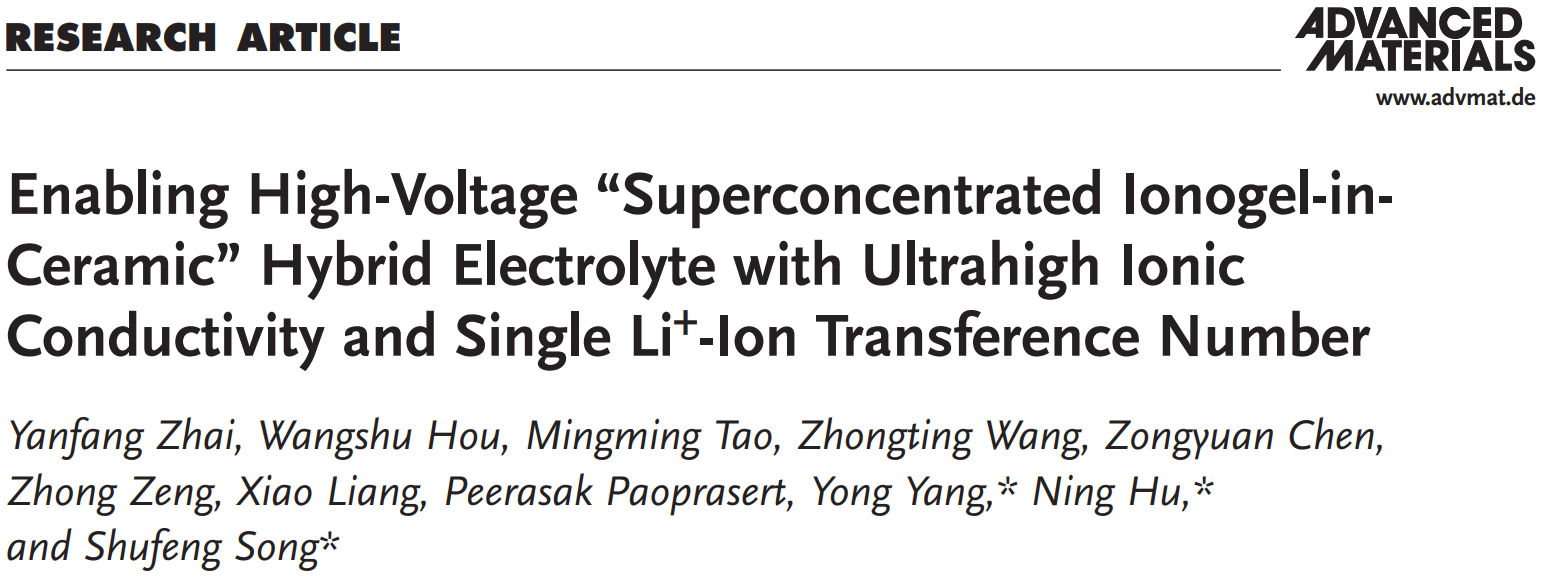
In this work, a high-voltage single-ion conducting “superconcentrated ionogel-in-ceramic” (SIC) novel hybrid electrolyte was enabled by conglutinating the garnet (LLZO) particles with a superconcentrated ionogel (3 M LiTFSI–EmimFSI–PMMA). An in situ polymerization method was proposed to address the issues of immiscibility barriers of polymers in ionic liquids in the ionogel preparation, and to solve the interfacial issues in the cell fabrication. Shown in the Figure 1 is the preparation process. The as-prepared SIC electrolyte displayed high heat tolerance in comparison with the commercial PP separator (Figure 1c).
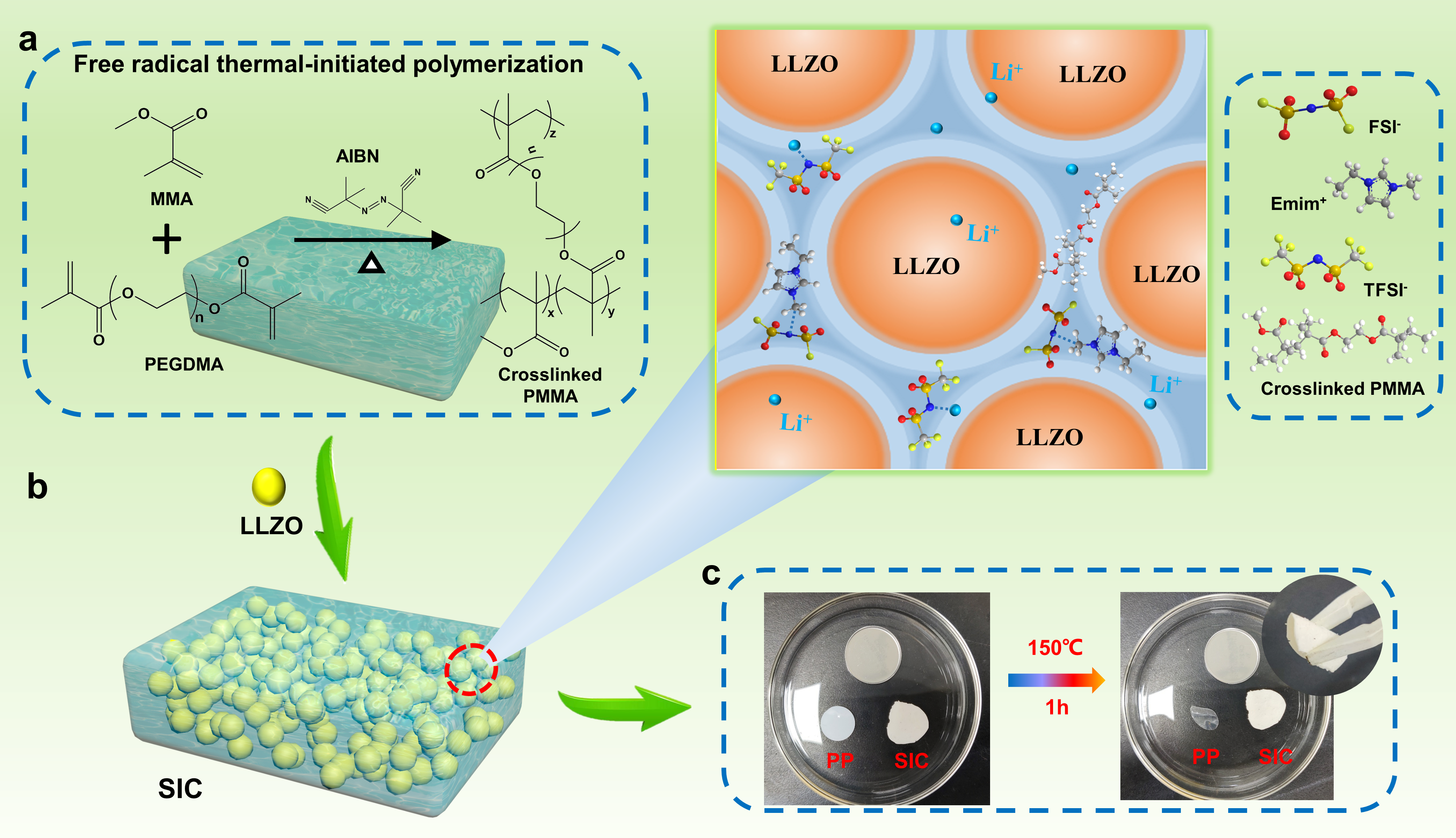
Figure 1. Schematic illustration showing the in situ synthesis of the SIC electrolyte. a) Thermally initiated radical polymerization of MMA monomer, PEGDMA crosslinker and AIBN. b) The network of the SIC electrolyte. c) The thermal stability of SIC electrolyte in comparison to the commercial polypropylene (PP) separator.
As one of the key components of the batteries, the electrolyte not only separates the cathode and anode, but also transports ions. The strong chemical interactions at the interface between the ceramic and the highly concentrated ionogel results in an integrated structure that allows the electrolyte to exhibit a high ionic conductivity of 1.33 × 10-3 S cm-1 at room temperature, which meets the practical application requirements of the electrolyte, and still has an ionic conductivity of 1.68 × 10-5 S cm-1 at -30 °C, which is better than most electrolyte materials. Moreover, the electrolyte has an electrochemical window of 5.5 V, an electron conductivity of only 3.14 × 10-10 S cm-1, and a Li+-ion transference number of up to 0.89. The combination of ultra-high ionic conductivity and large Li+-ion transference number makes this electrolyte promising for lithium metal battery applications.
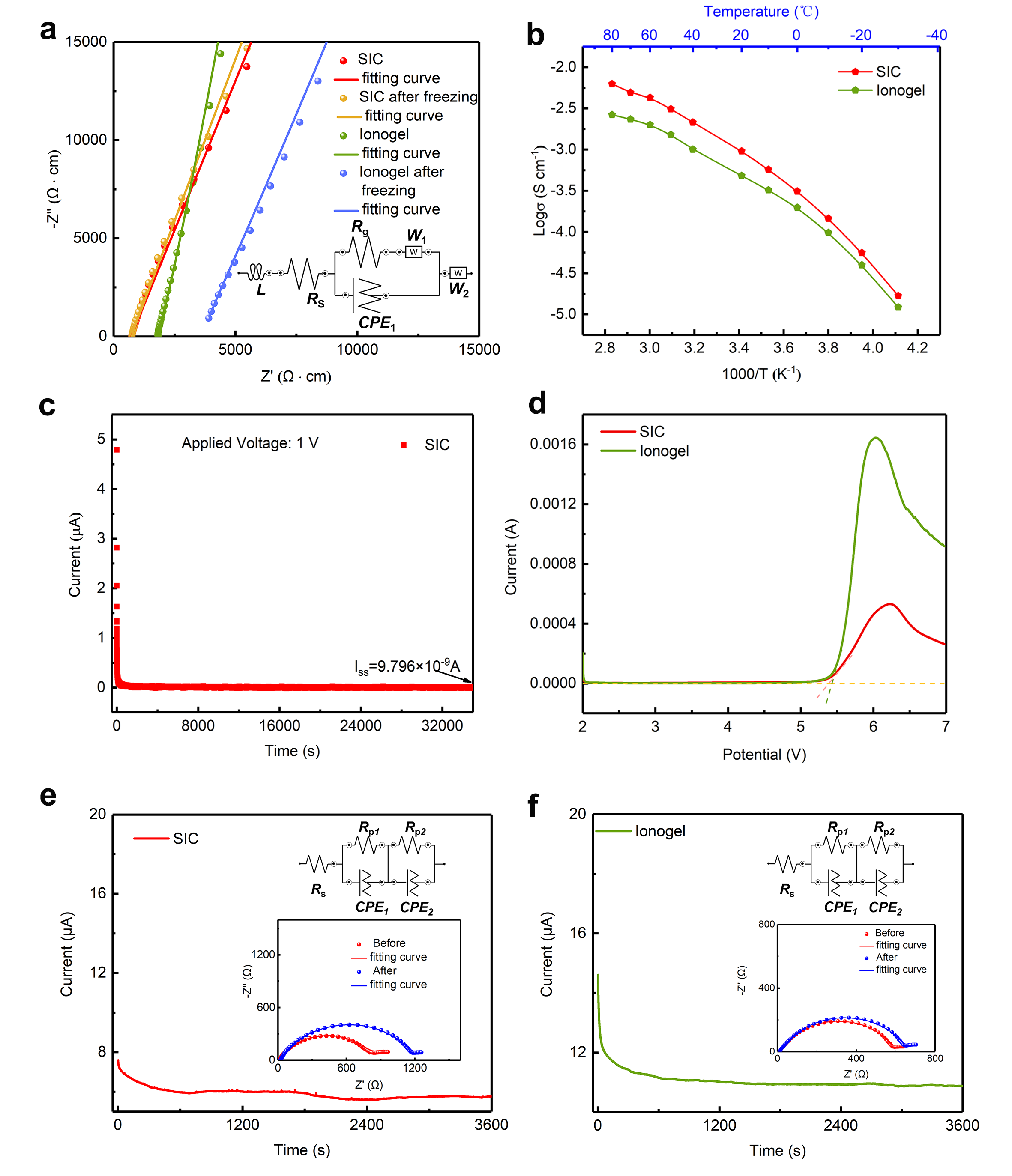
Figure 2. Electrochemical properties of the SIC and ionogel electrolytes. a) Electrochemical impedance spectroscopy of SIC and ionogel at 25 °C before and after freezing at −30 °C. The inset shows an equivalent circuit. b) Conductivity–temperature curves of SIC and ionogel in the temperature range of −30 °C to 80 °C. c) Direct-current polarization curve of SIC. d) Linear sweep voltammetry curves of SIC and ionogel. e,f) Current–time profiles of the Li symmetric cells with SIC and ionogel. The insets show equivalent circuit and impedance spectra of the batteries before and after polarization.
The issue of high interfacial resistance between electrolyte and electrode can be solved by forming the is-situ composite electrolyte via liquid precursors. Accordingly, the Li‖NCM523 and Li‖LiFePO4 solid-state batteries using the SIC electrolyte exhibited low resistance and stable electrolyte/electrode interphases. As demonstrated in Figure 3a,b, the Li||LiFePO4 cell using the SIC electrolyte, lithium-metal anode and LiFePO4 cathode was operated under a high rate of 1C at room temperature, and displayed a high Coulombic efficiency close to 100%. As demonstrated in Figure 3c,d, the Li||NCM523 cell using the SIC electrolyte, lithium-metal anode and LiNi0.5Co0.2Mn0.3O2 cathode was operated under a high cut-off voltage of 4.3 V, a high rate of 1C, and at a room temperature of 25 °C, and displayed favourable cycle performance, indicating the SIC can match with high voltage (4.3V) cathodes. As seen in Figure 3e, the SIC electrolyte and NCM523 were tightly integrated with an intact electrolyte/cathode interface. Notably, the LiTFSI–EmimFSI was infiltrated inside of the cathode as indicated by the EDS mapping of N and F elements. This phenomenon is a typical merit of in situ polymeric electrolyte that the precursor solution permeates into the electrode, leading to a low interface resistance. Thus, this "superconcentrated ionogel-in-ceramic" solid-state electrolyte offers a new pathway for high-safety, high-energy-density lithium metal batteries.
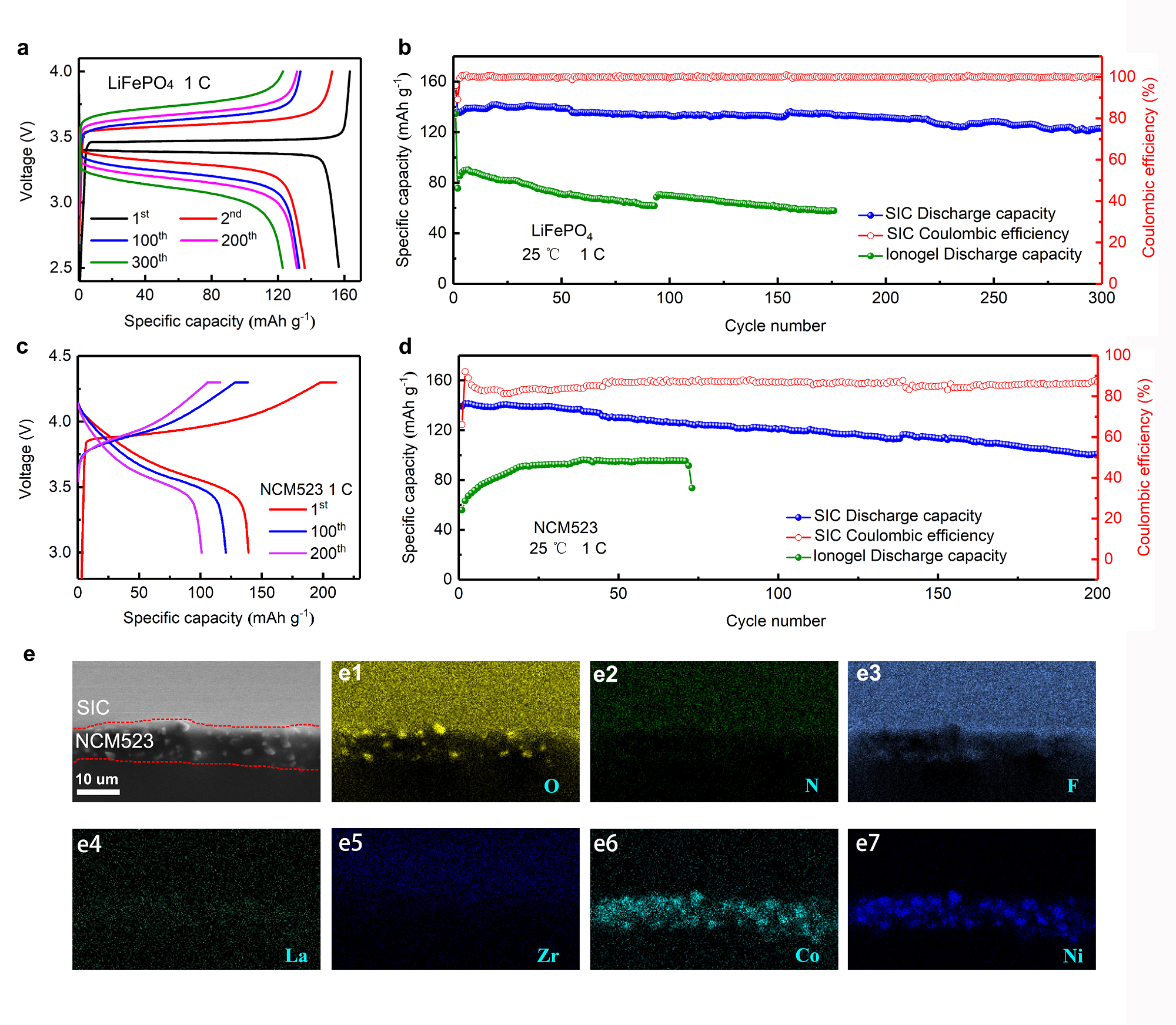
Figure 3. Electrochemical performance of the Li□||LiFePO4 and Li||NCM523 quasi-solid-state batteries with SIC and ionogel electrolytes under 1C at 25 °C. a) Voltage–capacity profiles of Li||LiFePO4 battery. b) Cycling performance of the Li||LiFePO4 batteries using SIC and ionogel. c) Voltage–capacity profiles of Li||NCM523 battery. d) Cycling performance of the Li□|| NCM523 batteries using SIC and ionogel. e) Morphologies and elemental compositions of SIC/NCM523 interface after cycling.
This is supported by Science and Technology Planning Project of Tianjin (20ZYJDJC00030), the Key Program of Research and Development of Hebei Province (202030507040009), the Fund for Innovative Research Groups of Natural Science Foundation of Hebei Province (A2020202002).
Article Link:https://doi.org/10.1002/adma.20220556