
The direct asymmetric reductive amination of heteroaryl ketones has been a long-standing synthetic challenge. Recently, Prof. Jiang Yanjun and Liu Yunting et al. achieved the engineering of an amine dehydrogenase (AmDH) from Jeotgalicoccus aerolatus for the asymmetric synthesis of chiral α-(hetero)aryl primary amines in excellent conversions (up to 99%) and enantioselectivities (up to 99% ee). The best AmDH variant ( Ja -AmDH-M33) exhibited high activity and specificity toward alkyl (hetero)aryl ketones, even for those bearing a bulky alkyl chain. An efficient directed evolution approach based on molecular docking was implemented to enlarge the active pocket with a more hydrophobic entrance, which is responsible for the high activity.
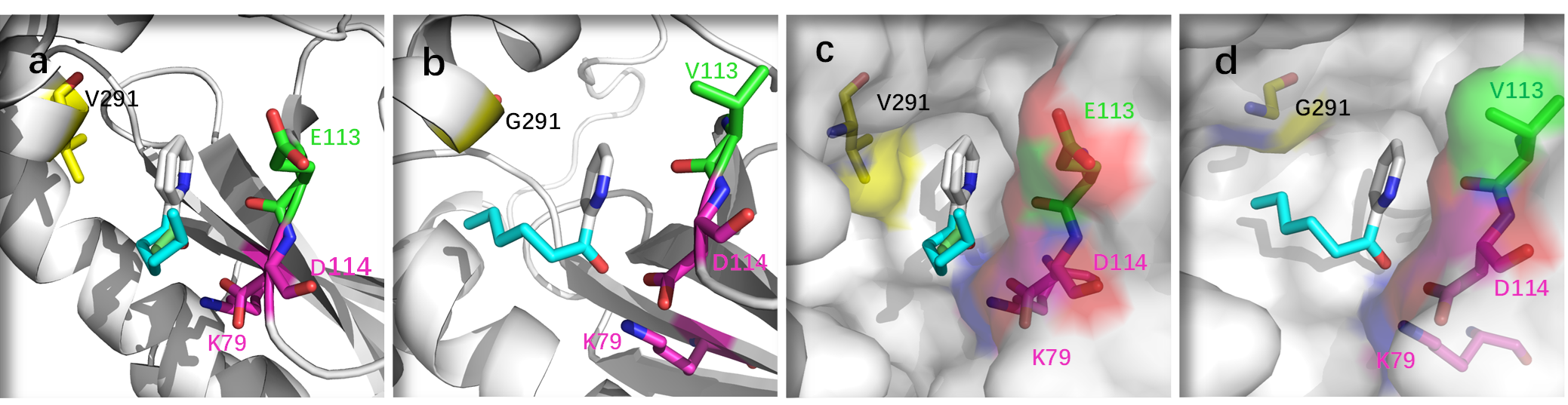
Under the optimized conditions, several critical synthons 15p, 17p, 18p, 23p and 26p to pharmaceutical and biologically active molecules were synthesized on a 100 mM scale in high yields (76-99%) and >99% ee. When the substrate concentrations were further increased to 1000 mM, the yields could still reach 67% and 71%, respectively, with promising TON values (more than 15000). This biocatalytic strategy has the advantages of both shorter steps and higher yields, confirming its potential for practical industrial applications, especially in the manufacture of pharmaceutical compounds.
