Research teams from Dr. Hailong An in Hebei University of Technology and Dr. Yingxian Li in China Astronaut Research and Training Center reveal the molecular mechanism of calcium-regulated chloride ion channel regulation for bone metabolism, and find a new target for the osteoporosis diagnosis and treatment.
Bone metabolic homeostasis plays an important regulatory role in the maintenance of body health. The bone metabolism imbalance caused by abnormal osteoclast function is an important inducement for osteoporosis and weightless bone loss. With the advent of an aging society, osteoporosis has become an important medical problem to the society. During the long-term manned spaceflights and deep space explorations via spaceflight projects in China, long-term in-orbit flight will cause bone metabolism disorders in astronauts. It is particularly necessary to reveal the molecular mechanism of abnormal osteoclast function which leading the imbalance of bone metabolism and discover new therapeutic targets.
The research teams from Dr. Hailong An in Hebei University of Technology and Dr. Yingxian Li in China Astronaut Research and Training Center recently published their 6-year research results in the journal Nature-Communications on May 24, 2022 with the title “Anoctamin 1 controls bone resorption by coupling CL- channel activation with RNAKL-RANK signaling transduction”. Their research reveals that calcium-regulated chloride ion channels (Anoctamin, Also known as ANO1) and osteoclast differentiation factor-mediated (RANKL-RANK) signaling pathway form a positive feedback loop to regulate osteoclast differentiation and bone metabolism. The ANO1 channel is expected to be a novel target for osteoporosis and weightless bone loss diagnosis and therapy.

Chloride ion, the most abundant anion in the body, plays an important role in the maintenance of cellular homeostasis. This study first reports the ANO1 is a key chloride channel in osteoclasts. Different with other chloride channels, the ANO1 channel enrolled in the process of osteoclast differentiation and bone resorption. The result demonstrates that the ANO1 channel promote the efflux of chloride ions and increase the secretion of protons to the dissolution of extracellular bone matrix in osteoclasts. In the same time, ANO1 directly interact with the key receptor proteins, promote the activation of the main signaling pathway, and initiate osteoclast differentiation. In addition, osteoclast differentiation factor up-regulate the expression of ANO1, activate phospholipase C-mediated calcium signal, further enhance the activity of ANO1 and its interaction with osteoclast differentiation factor receptor. The ANO1 and osteoclast differentiation factor-mediated signaling pathway forms a positive feedback loop in the differentiation process.
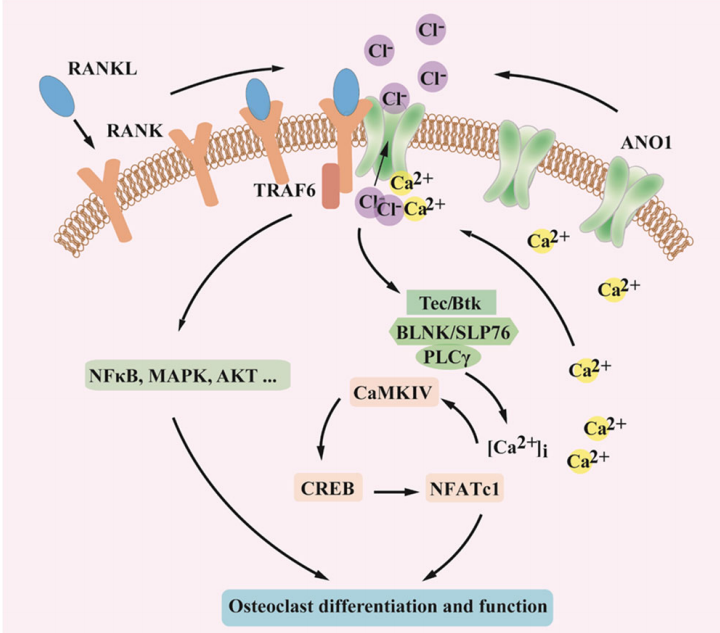
Molecular mechanism of ANO1 regulating osteoclast differentiation and activity
In vivo experiments demonstrated that osteoclast-specific ANO1 transgenic mice perform a decreased bone mass, enhanced osteoclast number and bone resorption capacity; In addition, osteoclast-specific ANO1 knockout mice had increased bone mass, decreased osteoclast number and bone resorption capacity. Knocking down the ANO1 gene significantly alleviated the occurrence of osteoporosis.
Further bone tissue samples from clinical osteoporosis patients shown that the elevated ANO1 expression in osteoporosis patients was closely related to the enhanced osteoclast function. Inhibition the ANO1 channel activity can significantly reduce osteoclast activity and bone resorption. Ion channel proteins are key drug targets and occupy an important position in drug research and development. The development of safe and effective inhibitors of calcium-regulated chloride ion channel ANO1 can be a new drug discover strategy for osteoporosis.


Dr. Shuai Guo, graduated from Hebut in 2020, Dr. Weijia Sun and Dr. Yuheng Li from the China Astronaut Research and Training Center are the co-first authors of the article. Professor Hailong An in Hebut, Yingxian Li and Shukuan Ling in CARTC are the co-corresponding authors. Professor Yong Zhan and Associate Professor Yafei Chen, Dr. Xuchao Wang, Dr. Jingxuan Fu, Dr. Sai Shi, Biao Ma, and Chang Qu are the co-authors. Dr. Hua Tian, director of the Department of Orthopedics, Peking University Third Hospital, and Dr. Cheng Wang, as co-authors, undertook the work related to the pathological analysis of bone tissue. The above work was completed under the funding of Key Project of the National Natural Science Foundation of China "Research on the regulation and mechanism of calcium-regulated chloride channels on weightless bone loss".
Original link: https://www.nature.com/articles/s41467-022-30625-9
